Abstract
T lymphoblastic leukaemia (T-ALL) is an aggressive lymphoid malignancy with adverse prognosis. The prognosis of relapse/refractory disease is dismal. Novel therapies for B lymphoblastic leukaemia such as blinatumomab and chimeric antigen receptor T-cell therapy are difficult to put into clinical use for T-ALL patients. Therefore, there is an urgent need to develop novel therapies for T-ALL.
Homoharringtonine (HHT) is a plant alkaloid derived from Cephalotaxus harringtonia tree which binds to ribosome and inhibits protein synthesis. FDA granted approval for HHT to treat chronic myeloid leukaemia in 2012. Combined HHT and FLT3 inhibitor is a powerful regimen in treating FLT3-ITD mutated acute myeloid leukaemia, with excellent side effect profile. Venetoclax is a BCL-2 inhibitor and has been known to be effective in various haematological malignancies. In addition to this, one study reported that combined venetoclax with MCL-1 inhibitor had synergistic effect in treating T-ALL.
Limited studies were conducted for evaluating the therapeutic effect of HHT on T-ALL so far from previous literature. No studies were conducted for investigating the role of combined HHT and venetoclax in treating T-ALL and it exhibits a major research gap in this area. To fill this research gap, we performed our study with the aims to: 1) Evaluate the therapeutic efficacy of HHT in T-ALL. 2) Delineate its mechanism of therapeutic effect in T-ALL. 3) Demonstrate the synergistic effect of combined treatment with HHT and venetoclax.
In this study, T-ALL cell lines namely KE-37, MOLT-4, LOUCY, CCRF-CEM were used. Cell viability of these cell lines was determined by trypan blue assay after treating with HHT for 24 and 72 hours. IC50 at 24- & 72-hours interval was calculated for all cell lines tested. Percentage of apoptotic cells was determined by flow cytometric analysis of PI and annexin V double staining. Western blot analysis of MCL-1 and BCL-2 proteins was performed. Cell lines were treated with combination therapy of HHT and venetoclax at various concentration. Combination index (CI) was determined for evaluating the synergistic effect.
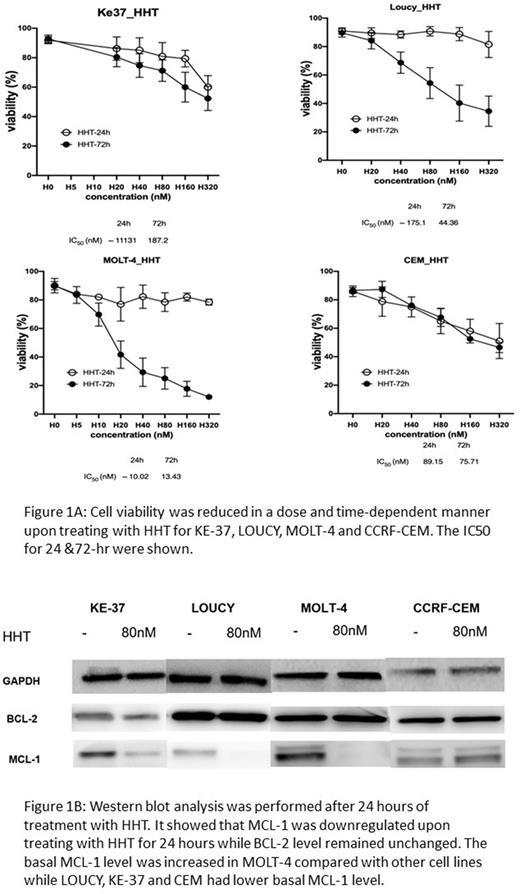
Our findings showed that cell viability was reduced upon treatment of HHT at time and dose-dependent manner. The percentage of apoptotic cells was also increased after treating with HHT in time and dose-dependent manner. The IC50 of HHT at 24- and 72-hours interval was shown in the figure 1A. In particular, HHT treatment was also active in LOUCY, a cell line with early T-cell precursor acute lymphoblastic leukaemia (ETP-ALL) phenotype. It implied that HHT was potentially effective to treat this aggressive subtype of T-ALL which had adverse prognosis.
Furthermore, western blot analysis showed that MCL-1 was downregulated upon treating with HHT for 24 hours. The level of BCL-2 remained unchanged (Figure 1B). MOLT-4 had a high basal MCL-1 level which also showed the lowest IC50 of HHT among all cell lines tested. KE-37, CEM and LOUCY had lower basal MCL-1 expression level and they showed a much higher IC50 of HHT compared with MOLT-4.
The percentage of apoptotic cells was increased upon combined treatment of HHT and venetoclax when compared with single treatment alone and control group. Synergistic effect was observed upon combined treatment of HHT and venetoclax with CI <1 in all cell lines tested.
Our study provides preliminary evidence that HHT is effective in treating T-ALL through downregulation of MCL-1. Moreover, it is reasonable to postulate that MCL-1 overexpression confers sensitivity towards HHT treatment from our preliminary findings. Combination treatment of HHT and venetoclax achieved synergistic effects. This combination regimen was also effective in promoting apoptosis in LOUCY cell line which had ETP-ALL phenotype with CI less than 1. Hence, our findings are potential to be translated into an effective treatment to this aggressive subtype of T-ALL and improve its clinical outcome. Further studies by in-vivo model will be performed to ascertain the clinical efficacy of this combination regimen in treating T-ALL. Moreover, the underlying mechanism of MCL-1 downregulation upon HHT treatment in triggering apoptosis needs further exploration. Nevertheless, our study provides a solid ground work of establishing the role of HHT and its combination with venetoclax in treating T-ALL.
Acknowledgements: The work was supported by Health and Medical Research Fund (Project No: 07182526).
Disclosures
No relevant conflicts of interest to declare.
OffLabel Disclosure:
Homoharringtonine. Currently the drug was grant by FDA to be administered in patients with chronic myeloid leukaemia. Here, we will discuss the study to show preliminary data of this drug to be used in acute lymphoblastic leukaemia.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal